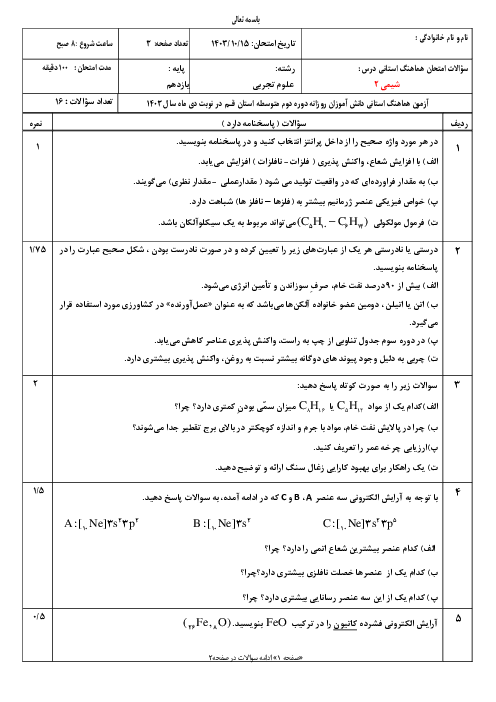
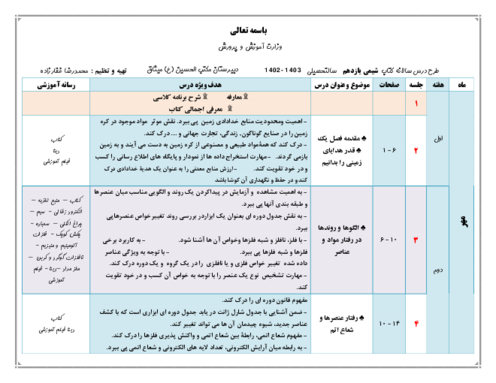
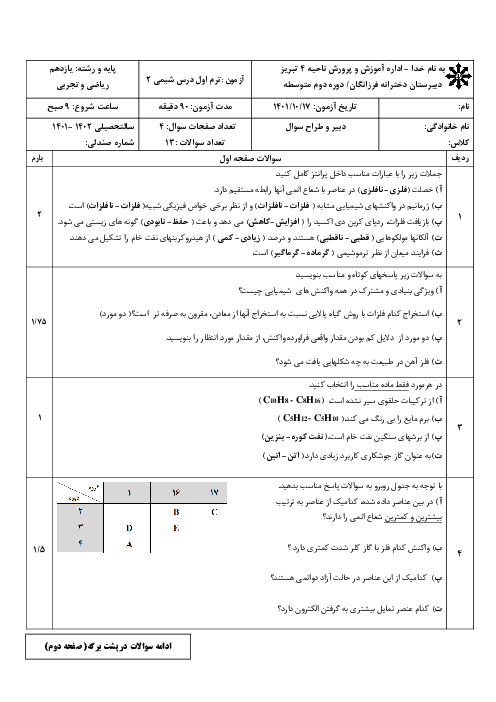
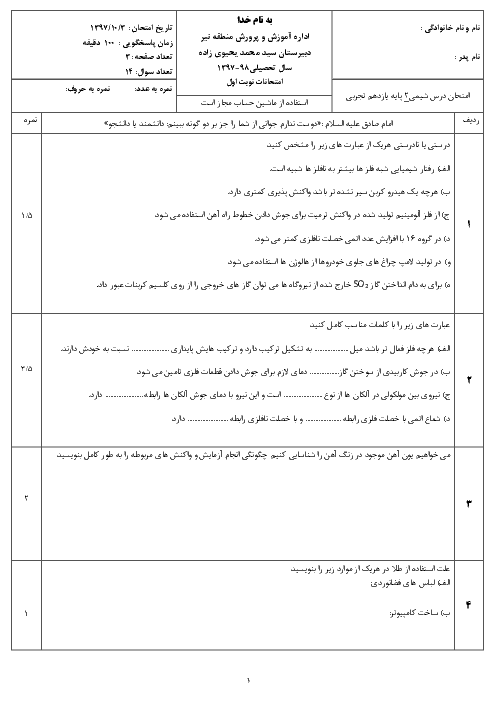
$\begin{array}{r}\mathrm{C}_4 \mathrm{H}_8 \mathrm{O}_2+\mathrm{C}_2 \mathrm{H}_6 \mathrm{O} \rightarrow \mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_2+\mathrm{H}_2 \mathrm{O} \\ ? \mathrm{gCC}_4 \mathrm{H}_8 \mathrm{O}_2=11 / 6 \mathrm{gC}_6 \mathrm{H}_{12} \mathrm{O}_2 \times \frac{1 \mathrm{molC}_6 \mathrm{H}_{12} \mathrm{O}_2}{116 \mathrm{gC}_6 \mathrm{H}_{12} \mathrm{O}_2} \\ \times \frac{1 \mathrm{molC}_4 \mathrm{H}_8 \mathrm{O}_2}{1 \mathrm{molC}_6 \mathrm{H}_{12} \mathrm{O}_2} \times \frac{88 \mathrm{~g} \mathrm{C}_4 \mathrm{H}_8 \mathrm{O}_2}{1 \mathrm{molC}_4 \mathrm{H}_8 \mathrm{O}_2} \times \frac{100}{80}=11 \mathrm{gC}_4 \mathrm{H}_8 \mathrm{O}_2\end{array}$