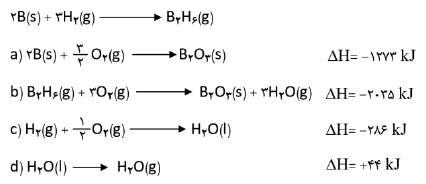
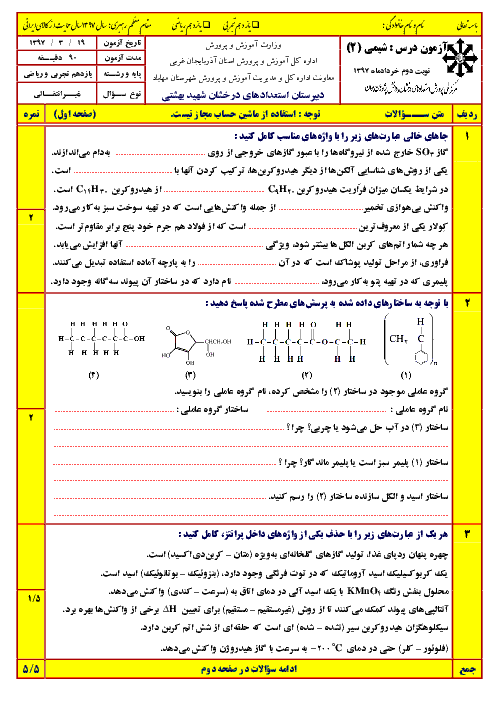
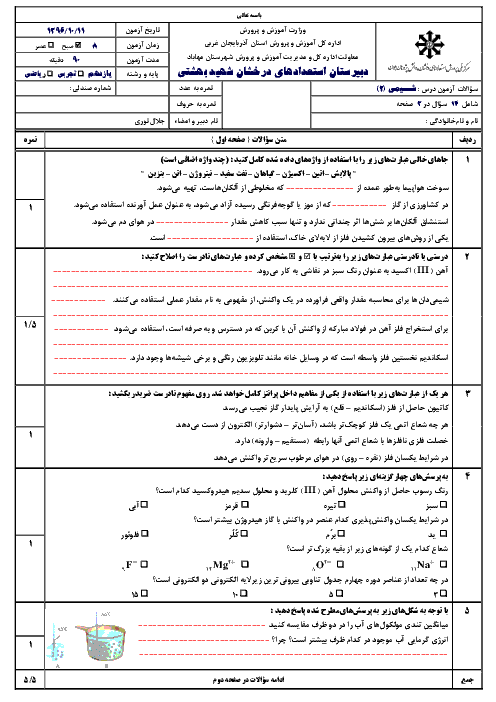
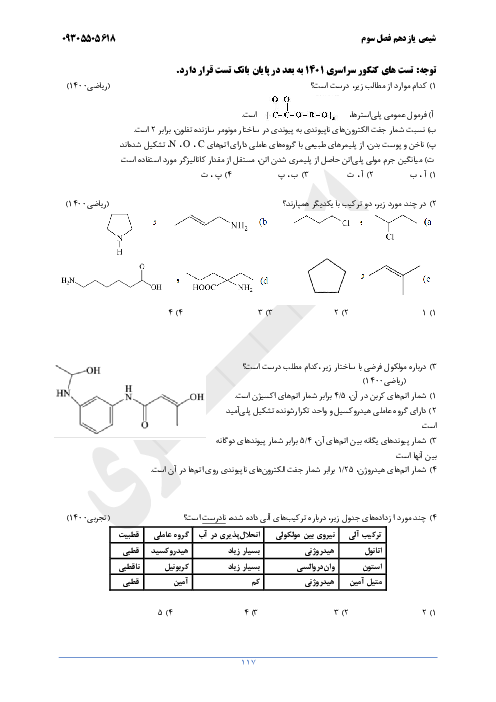
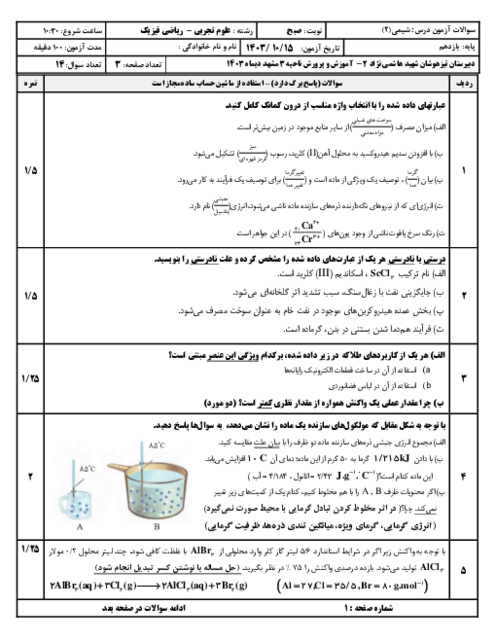
$a)2B(s) + \cancel{{\frac{3}{2}{O_2}}}(g) \to \cancel{{{B_2}{O_3}}}(s)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H = - 1273kJ$
$b)\cancel{{{B_2}{O_3}}}(s) + 3H\cancel{{_2O}}(g) \to {B_2}{H_6}(g) + 3\cancel{{{O_2}}}(g)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H = + 2035kJ$
$c)3{H_2}(g) + \frac{3}{2}\cancel{{{O_2}}}(g) \to 3H\cancel{{_2O}}(l)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H = - 858kJ$
$d)3\cancel{{{H_2}O}}(l) \to 3\cancel{{{H_2}O}}(g)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H = + 132kJ$
$2B(s) + 3{H_2}(g) \to {B_2}{H_6}(g)\,\,\,\,\,\,\,\Delta H = ( - 1273) + ( + 2035) + ( - 858) + ( + 132)kJ = 36kJ$
$69g\,{B_2}{H_6} \times \frac{{1mol\,{B_2}{H_6}}}{{27/6g\,{B_2}{H_6}}} \times \frac{{36kJ}}{{1mol\,{B_2}{H_6}}} = 90kJ$