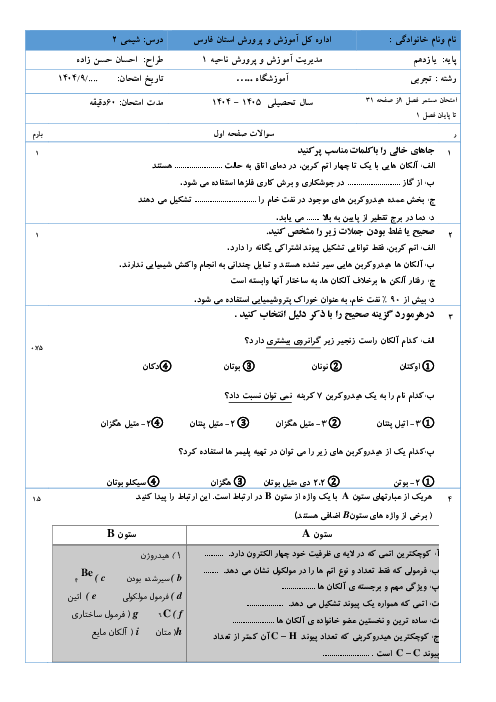
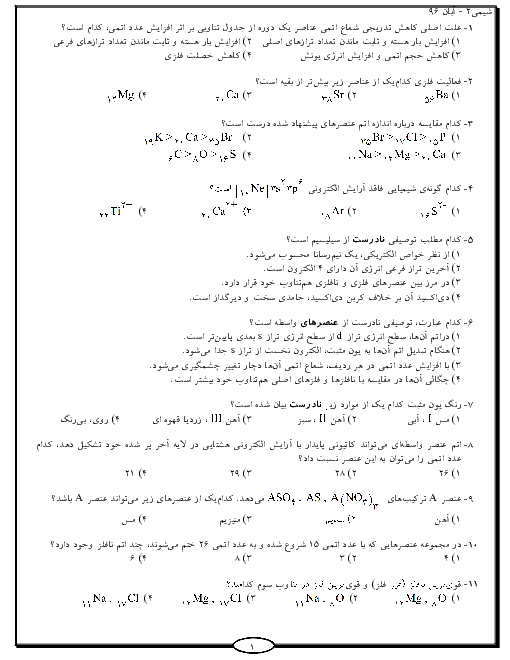
با استفاده از دادههای زیر، مقدار $\Delta H$ واکنش $C{{S}_{2}}(l)+6{{H}_{2}}{{O}_{2}}(l)\to C{{O}_{2}}(g)+6{{H}_{2}}O(l)+2S{{O}_{2}}(g)$ برحسب کیلوژول کدام است؟
$C{{O}_{2}}(g)+2S{{O}_{2}}(g)\to C{{S}_{2}}(l)+3{{O}_{2}}(g)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H=1077kJ$
${{H}_{2}}(g)+{{O}_{2}}(g)\to {{H}_{2}}{{O}_{2}}(l)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H=-188kJ$
${{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\to {{H}_{2}}O(l)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\Delta H=-286kJ$