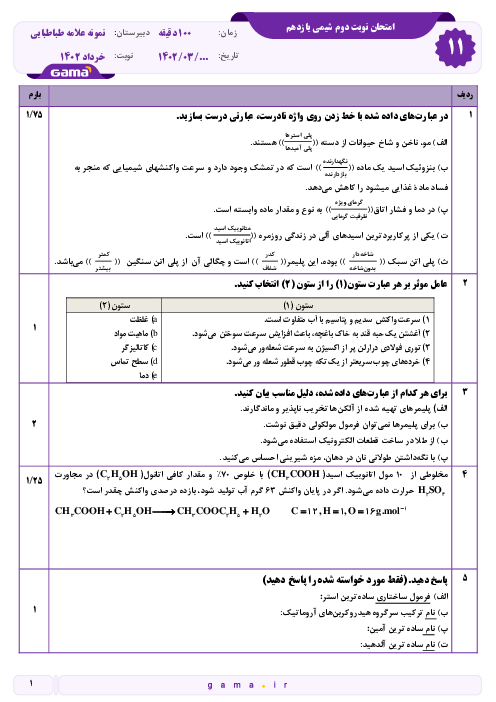
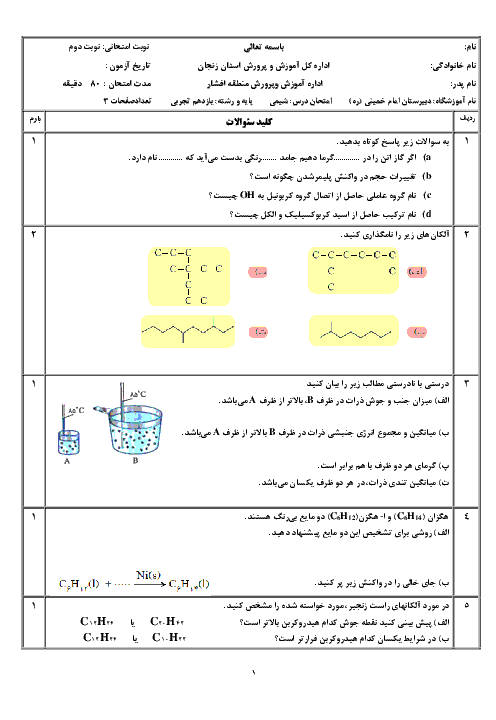
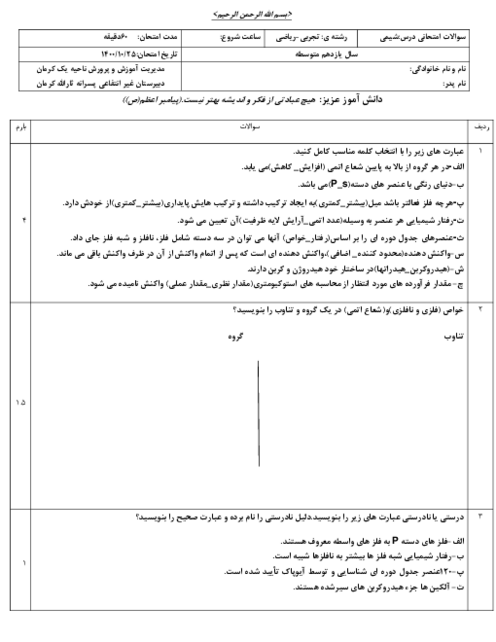
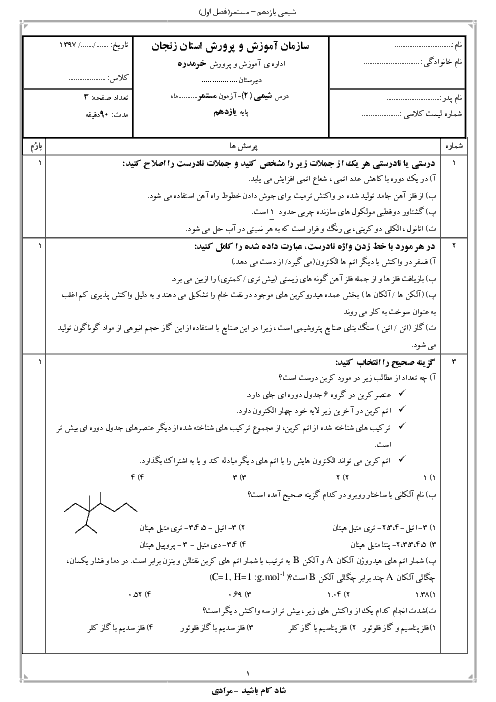
بر اساس اطلاعات داده شده $\Delta H$ واکنش ${{N}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\to {{N}_{2}}O(g)$ برحسب کیلوژول چقدر است؟
$\begin{align}
& 1)\,{{C}_{(S,graphite)}}+{{N}_{2}}{{O}_{(g)}}\to C{{O}_{(g)}}+{{N}_{2(g)}}\,\,\,\,\,\,\,:\Delta H=-193KJ \\
& 2)\,{{C}_{(S,graphite)}}+{{O}_{2(g)}}\to C{{O}_{2(g)}}\,\,\,\,\,\,\,\,\,\,\,\,\,:\Delta H=-394KJ \\
& 2C{{O}_{(g)}}+{{O}_{2(g)}}\to 2C{{O}_{2(g)}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,:\Delta H=-566KJ \\
\end{align}$
1 )
304-
2 )
98
82
4 )
21-